Creating a Reliable Plasma Ecosystem in Europe
Why plasma is essential
Plasma-derived medicinal products (PDMPs) treat many rare diseases and critical medical conditions, many of which are genetic and hereditary in nature, including haemophilia, Alpha-1 Antitrypsin deficiency, and immune deficiencies. Without PDMPs, many patients might not survive or may have a substantially diminished quality of life. Moreover, thanks to the incredible development of modern medical science PDMPs can help a much wider group of patients, such as those suffering from cancer and treated with chemotherapy.
130
Plasma donations to treat ONE primary immune deficiency patient for one year.
900
Plasma donations to treat ONE Alpha-1 antitrypsin deficiency patient for one year.
1200
Plasma donations to treat ONE haemophilia patient

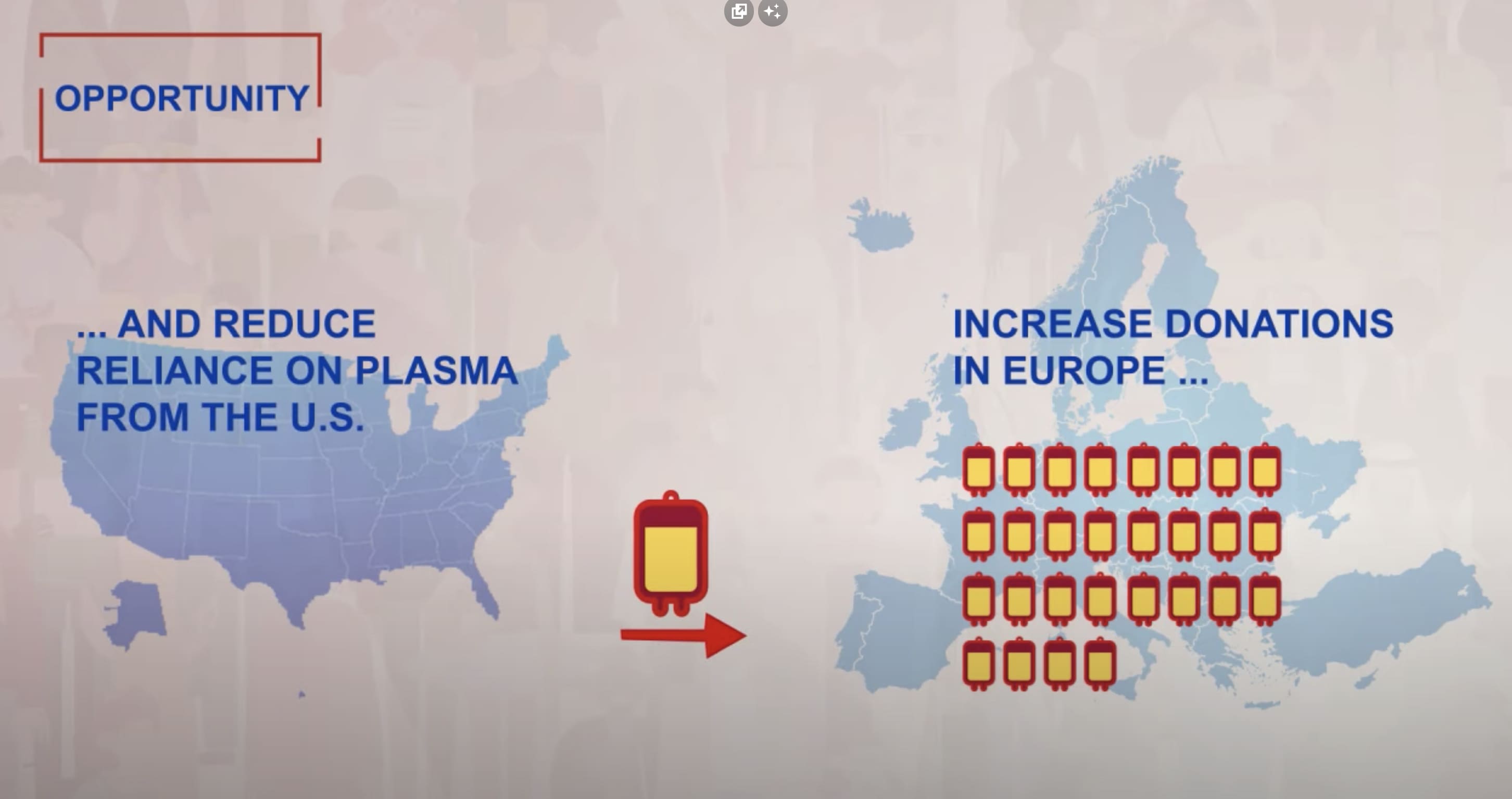
The eu needs more plasma

Every year, more and more patients across the EU are diagnosed with life-threatening plasma protein-related disorders. Those include immune deficiencies, alpha-1 antitrypsin deficiencies, hemophilia, and other bleeding disorders. In most cases involving these rare diseases, the only effective treatment option comes in the form of plasma-derived medicines.
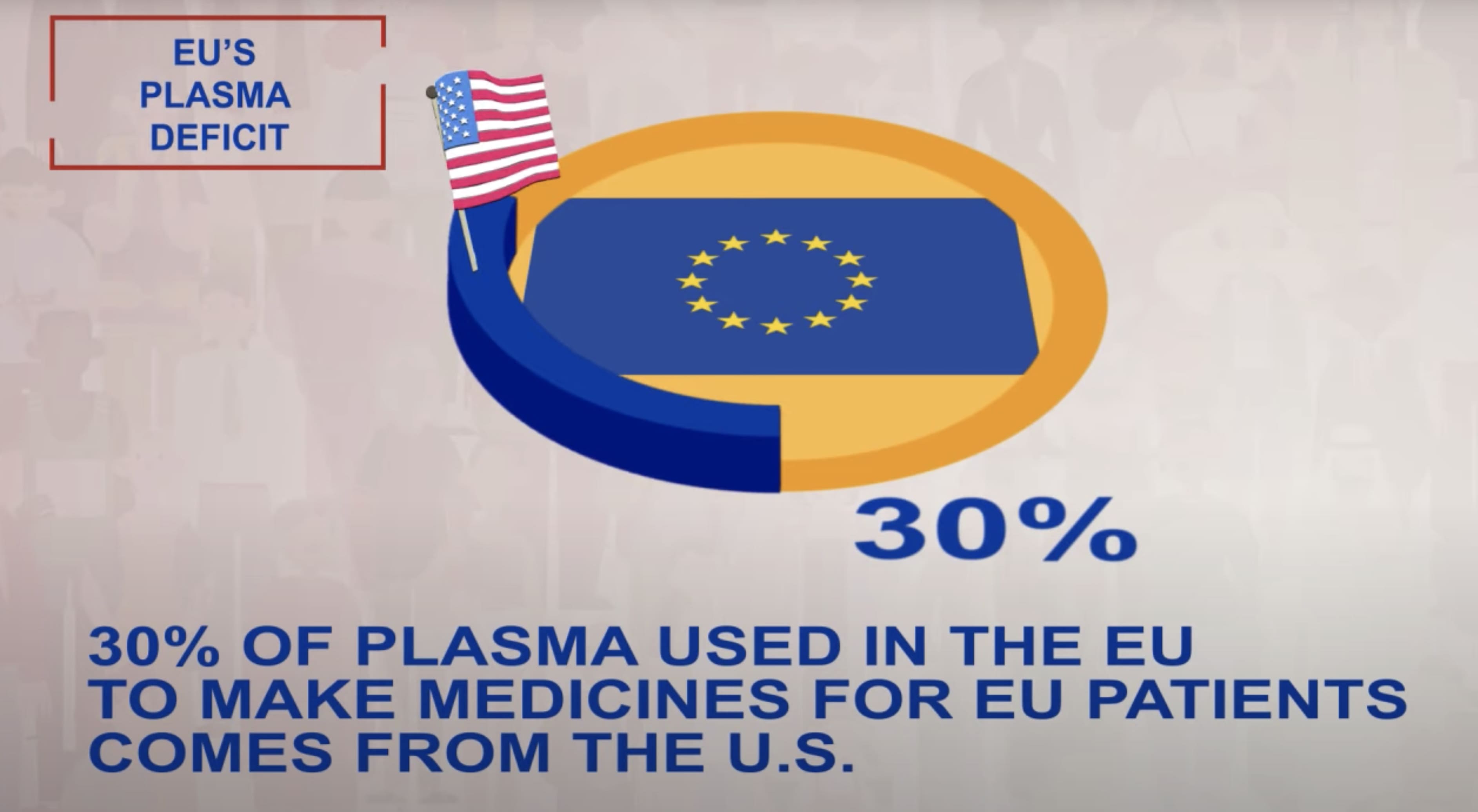
Over the past 10 years, the use of immunoglobulins (IG) – some of the most used plasma-derived medicines – has almost doubled. Innovations in medical research and consecutive efforts to increase immunoglobulin usage across European countries contribute to a growing clinical need for the plasma-derived medicines, with 40% of plasma used for manufacture being sourced from the U.S.

DID YOU KNOW?
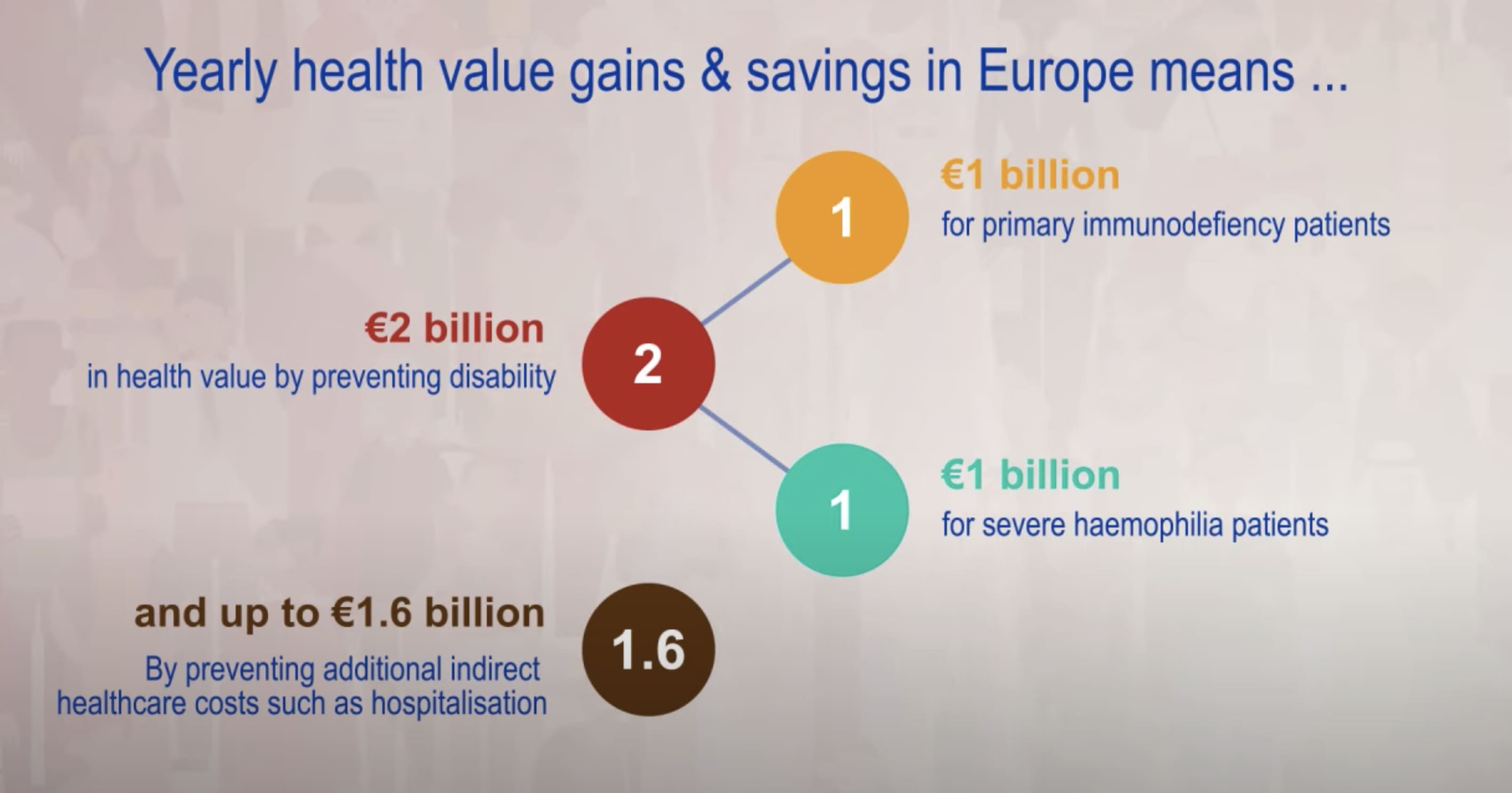
300,000 patients in the European Union rely on plasma-derived medicines to manage rare diseases such as immune deficiencies, bleeding disorders, inflammatory conditions, and neurological diseases.
Almost all plasma-derived medicines are on the EU’s List of Critical Medicines.
Videos
Find out more about the challenges posed by the EU’s current reliance on plasma from non-EU countries.
REGULATION & LEGISLATION
In order to ensure regular access to plasma medicines for those in need, it is critical that policymakers acknowledge the clinical urgency for increased plasma collection in Europe by putting in place the most appropriate EU and national policy frameworks.
SoHO regulation
SoHo regulation refers to the European Commission’s proposal on standards of quality and safety for substances of human origin. The new regulation aims to improve patient access to high-quality plasma-derived medicinal products (PDMPs), with consideration for the health and safety of donors and some 300,000 EU patients that rely on these therapies.
EU pharmaceutical legislation
The European Commission’s revision of the EU pharmaceutical legislation presents a much-needed opportunity to address the need for equal EU patients’ access to safe, state-of-the-art, and affordable medicines, including PDMPs, to treat and prevent diseases, as well as the need to support and foster innovation in the EU.

IPAW Updates
Get Social
Follow PPTA on social media to stay connected during #IPAW2024.














.png)
.png)
.png)
.png)
.png)

.avif)